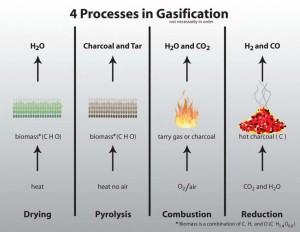
What takes place in a gasification reactor?
The concept of converting waste to energy to help us heat and power our daily lives is pretty amazing. If we think back 50 years ago, the environmental movement was barely a blip on the radar. Now being environmentally friendly is not just a trend but an expectation. Technology has come a long way in the past 50 years and thanks to those advances we have systems that can take the wind, the sun or our daily garbage and convert it to electricity, heat, and steam to power the world. So just how does a waste to energy technology like downdraft gasification work? What goes on inside the gasification reactor to turn our banana peels and lawn clippings into a gas (known as syngas) that runs a generator?
As our CEO at Waste to Energy Systems likes to describe it, it is like the oxygen two-step; there are only a few oxygens at the party and everyone wants to dance. This is due to the fact that the partial combustion of the fuel for the gasification process takes place in an oxygen deprived environment. For this discussion, we will use wood chips as our fuel. When the wood chips drop in the reactor, it enters the first stage, the drying stage.The wood chips enter an area of 200 F, where any moisture left in the wood chips is driven off and turned into steam.
After the moisture is successfully removed, it enters the pyrolysis stage. Here the fuel is exposed to higher heat levels and no air is available. The heat and lack of oxygen causes the fuel to begin breaking down. What is left from this stage is charcoal, gas, a sticky substance called tar, and some liquids.
As the newly formed gasses, liquids, and solids continue down the rabbit hole, they end up in the combustion stage, where things really heat up. Air is added back in at this point to create temperatures so high that they burn off any unwanted elements in the fuel stream. We are talking temperatures up to 2200 F! That is equivalent to the heat needed to forge steel.
Then its on to the reduction stage, where the oxygen do si do really begins. The combustion stage has broken everything down to the basics and it is a battle between carbon and hydrogen for the oxygen atoms attention. In the end, the oxygen is more interested in the carbon and they become the final dance partners. So, we are left with carbon monoxide and hydrogen, which are the main components of syngas (the goal of gasification). Both of these gases are highly combustible and can easily power a generator to create electricity.
Without every stage of the gasification reactor, systems like our bioHearth downdraft gasification unit would not be successful. Gasification is a simple and effective technology that will only become higher in demand as our population grows. It is a great way to create a renewable, alternative energy system!